Spray drying is a very widely used technical method for drying aqueous or organic solution, emulsion and so on in industrial chemistry and food industry. Milk powder, detergents and dyes are just a few of the spray-drying products currently available. Spray drying can be used to preserve food or simply as a quick drying method and has the advantage of reducing weight and bulk. This is done by spraying the feed into a hot drying medium to change the feed from a fluid state to a dry granular form. Over the past two decades, after intensive research and development, spray drying has become a highly competitive method of drying a wide range of products. The range of applications continues to expand, so today spray drying is relevant to many of the things we use every day.
2. Principle of spray drying
By mechanical action, the material to be dried is dispersed into very fine particles like fog, (increase the evaporation area of water, speed up the drying process) and hot air contact, in an instant to remove most of the water, so that the solid substance in the material dry into powder, and then the product and air separation. The mixture sprayed may be sol, emulsion, suspension or dispersion.
2.1 Atomize the feeding solution into small particles
The whole process of spray drying basically consists of four processes:
Atomization can be achieved by pressure nozzles, two-fluid nozzles, rotating disk atomizers, or ultrasonic nozzles. Therefore, different kinds of energy can be used to disperse the liquid into fine particles. The choice of atomizer type depends on the nature and amount of feed and the desired characteristics of the dried product. The higher the dispersed energy, the smaller the droplets.
Nozzles and products
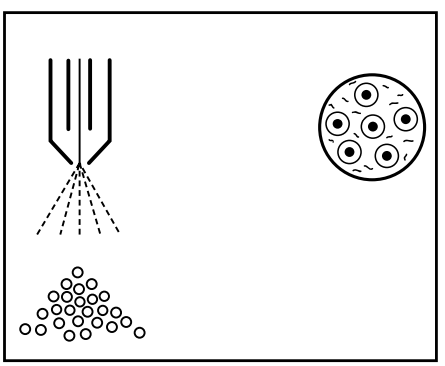
Example: Spraying 100 ml of solution produces about 8 x 108=800000000 drops (25 microns), representing about 12 m2 of surface area. This clearly shows that the solution (mainly water) evaporates very quickly.
2.2 Mixing of spray and drying medium (air) -- a process of mass and heat transfer
The way the spray contacts the dry air is an important factor in the design of the spray dryer, as this has a great influence on the properties of the dried product by affecting the behaviour of the droplets during drying. Method of mixing is an important consideration and defines the method of spray drying:
Downstream
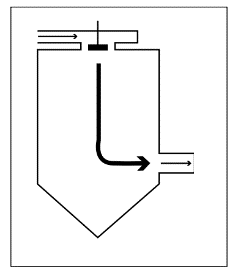
The material is sprayed in the same direction as the hot air flow through the equipment. The droplets come into contact with hot, dry air when they are at their wettest. Because the material evaporates instantly, the product should be handled carefully during drying.
Counter current
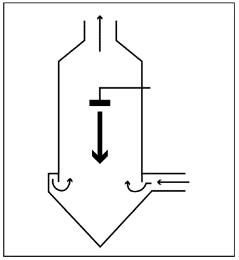
The material is sprayed in the opposite direction to the hot air flow. The hot air flows upward, and the product passes through the hotter air to fall into the collection tray. The residual water evaporates and the product becomes very hot. This method is only applicable to products with high thermal stability.
Mixed flow
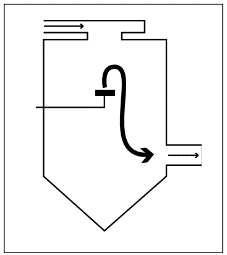
Combines the advantages of both spray methods. The product is sprayed upward and only stays in the hot area for a short time to evaporate residual water. Gravity then pulls the product into the cooling zone. The product should be handled with care as it only stays in the hot area for a short time.
Rotary atomizer (rotary disk)
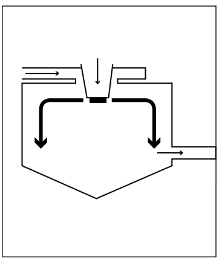
The material to be atomized flows onto a rapidly rotating atomizing disk and is transformed into a fine mist. Dry air flows in the same direction. As with the downstream method, handle the product with care.
2.3 Open circulation system and closed circulation system
Air is primarily used as a drying medium. The air flow is heated electrically or in a burner, and after this process is discharged into the atmosphere. It's an open loop system. If the heating medium is recycled, usually an inert gas such as nitrogen, it is a closed cycle system. These layouts are often chosen when dealing with flammable solvents, toxic products, or oxygen sensitive products. The most common type of spray dryer is the open cycle downflow spray dryer. In this design, atomized feed and dry air are injected into the spray drying chamber simultaneously from the same direction.
2.4 Spray drying (water evaporation)
Once the spray droplets come into contact with dry air, they evaporate from the saturated vapor film that quickly forms on the droplet's surface. Due to the high specific surface area and existing temperature and humidity gradients, the intense mass and heat transfer process results in efficient evaporative drying. Evaporation causes the droplet to cool, resulting in a small heat load. The design of the drying chamber and the air flow rate provide the amount of time the droplets remain in the drying chamber to complete the desired drop moisture removal and remove the product from the dryer before the product temperature rises to the outlet dry air temperature. In this way, there is little chance of thermal damage to the product.
2.4.1 Separation of product and air
In principle, two systems are used to separate the product from the drying medium:
1 The primary separation of dried products is carried out at the bottom of the drying chamber
2. Total recovery rate of dried products in the separation equipment
The most common separation device is a cyclone. Based on inertial forces, the particles are separated to the cyclone wall as a downward strain and removed. Other systems include wet collectors such as electrostatic precipitators, fabric (bag) filters or scrubber.
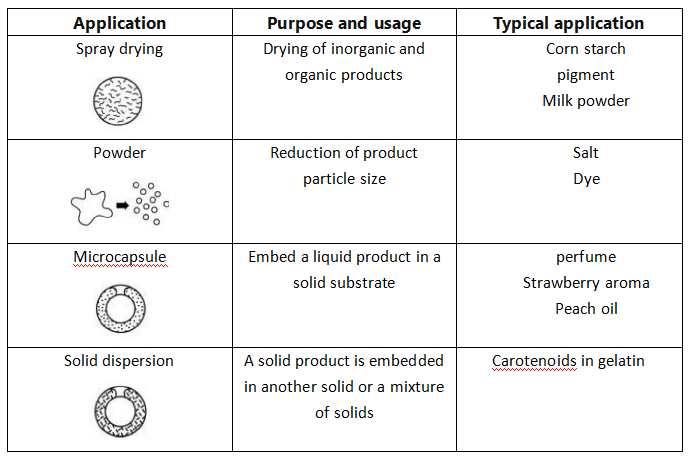
3. Common applications
There are many materials that can be successfully spray dried. Only the general principles are listed here
Step 4 Apply
4.1 Spray Drying
Spray drying is suitable for most aqueous or colloidal solutions, for emulsions and dispersions, as long as the product behaves as a solid after drying.
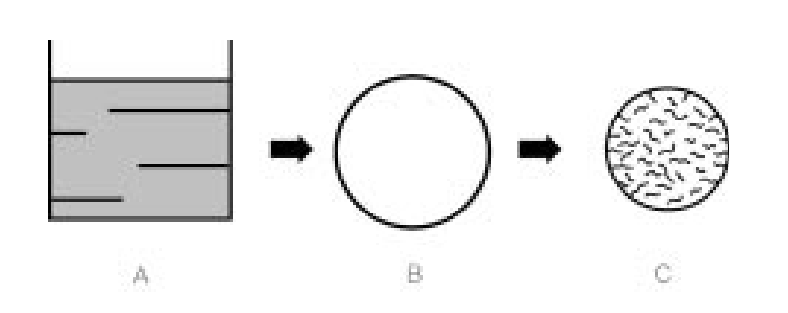
Schematic diagram of spray drying inorganic or organic products
A two-fluid nozzle is used to disperse the aqueous solution of the product (A) into extremely fine spray droplets (B). The solution immediately evaporates around the product, forming a vapor cloud that protects the product from thermal load. Once the critical concentration is exceeded, nucleation begins, forming a solid shell. After water dries from the surface, the interface enters the core (the second step of drying). The end product (C) is a fine, amorphous or crystalline material. Highly concentrated spray solutions produce porous end products.
Actual case
| Product inlet temperature ℃ Outlet temperature ℃ Spray concentration % |
|
Food Low fat milk 174 102 50 Yeast 95 55 60 |
|
Perfume/cosmetics Beer concentrate 150 110 30-40 Olive leaf extract 150 90 36 |
|
Medical/pharmaceutical Plasma 180 100 5 Polypeptide 110 70 2 |
|
Chemical products Disperse dye 150 95 20 |
4.2 Micropulverization or structural changes
Micropulverization or structural change is a change in morphology, for example, if a fine powder is required. This has a positive effect on the solubility or measurability of the final product. The main advantage of micropulverization is that very regular particle sizes are achieved.
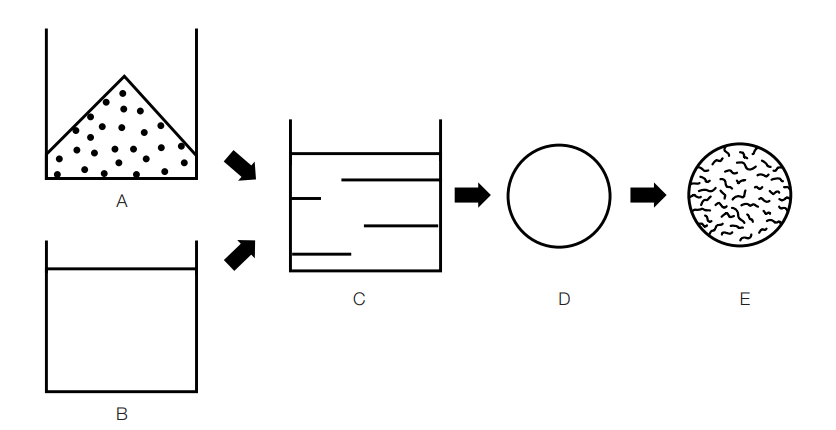
Diagram of the process of micronization or structural change
Dissolve the crystalline product (A) in a solvent (B) and disperse the solution (C) into small droplets (D). The result is a final product (E), like the final product described in the spray drying section.
Actual case
| Product inlet temperature ℃ Outlet temperature ℃ Spray concentration % |
|
Food Lactose 160 105 30 Corn starch 130 70 40 |
|
Perfume/cosmetics Metal soap 165 122 60 Detergent 200 110 40 |
|
Medical/pharmaceutical Fructose-amino acid compound 180 80 37 |
|
Chemical products Calcium carbonate 220 100 10 Sodium citrate 160 90 20 |
4.3 Microencapsulation
Schematic diagram of microencapsulation process
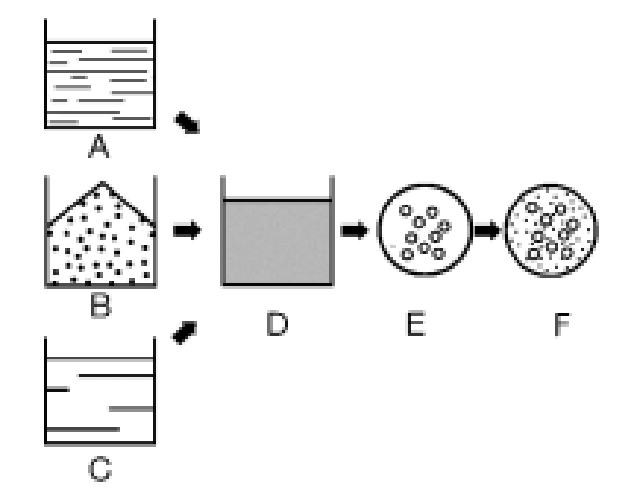
An emulsion (D) is formed in water from A liquid product to be treated (A), a carrier substance (B) such as maltodextrin and a film forming agent solution (C) such as gum acacia. The emulsion is then atomized into small droplets (E). The solvent evaporates, resulting in dispersion of the second phase (F) around the solid matrix. The result is that small droplets of the product (A) are stored in the carrier substance (B) and embedded in the film forming agent (C).
Actual case
| Product inlet temperature ℃ Outlet temperature ℃ Spray concentration % |
|
Food Maltodextrin/ Gelatin Soybean Oil 150 90 30 |
|
Perfume/cosmetics Maltodextrin/ Arabic gelatin medium 150 90 35 Strawberry fragrance 1.5:1.5:3 |
|
Medical/pharmaceutical Medical/pharmaceutical 120 70 ca. 30 Squalene1:2:1 |
4.4 Solid dispersion
Diagram of solid dispersion process
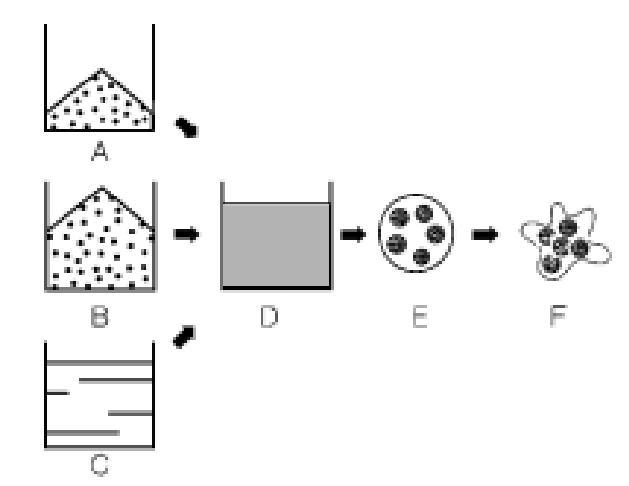
The evaporation process is similar to the microencapsulation process, which uses solid materials instead of liquid products. Form A solution or dispersion (D) from the product to be treated (A), the substrate (B), and eventually water with additional film forming agent (C). The solution is then atomized into small droplets (E). Substrate and/or film forming agent causes agglomeration or coating of suspended particles (F).
Actual case
| Product inlet temperature ℃ Outlet temperature ℃ Spray concentration % |
|
Food Invert sugar in lactose 100 80 2 (jujube pulp)1:1 |
|
Perfume/cosmetics Low fat milk powder/glucose / 90 70 40 Streptococcus in gelatin 1:1:1:3 |








.jpg)


.jpg)
.jpg)

.jpg)


